 The +I effect of −CH3 group increases the electron density on the O−H bond. Therefore,
The +I effect of −CH3 group increases the electron density on the O−H bond. Therefore,
release of proton becomes difficult. On the other hand, the −I effect of F decreases the
electron density on the O−H bond. Therefore, proton can be released easily. Hence,
CH2FCO2H is a stronger acid than CH3CO2H.
 F has stronger −I effect than Cl. Therefore, CH2FCO2H can release proton more easily than
F has stronger −I effect than Cl. Therefore, CH2FCO2H can release proton more easily than
CH2ClCO2H. Hence, CH2FCO2H is stronger acid than CH2ClCO2H.

Inductive effect decreases with increase in distance. Hence, the +I effect of F in
CH3CHFCH2CO2H is more than it is in CH2FCH2CH2CO2H. Hence, CH3CHFCH2CO2H is stronger acid than CH2FCH2CH2CO2H.
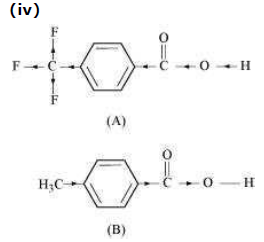
Due to the −I effect of F, it is easier to release proton in the case of compound (A). However, in the case of compound (B), release of proton is difficult due to the +I effect of −CH3 group. Hence, (A) is a stronger acid than (B).